- RWD
Unicancer provides support to expert groups responsible for addressing questions on therapeutic strategies and research.

PROMOTING AND DEVELOPING INNOVATIVE AND STRATEGIC CLINICAL RESEARCH TO IMPROVE THE TREATMENT OF PATIENTS WITH BREAST CANCER.
Since 1994, the Unicancer Breast group is the national referent cooperative group in its field. In 2013, following its partnership with ARCAGY-GINECO cooperative group, it was renamed the Unicancer French breast intergroup (UCBG). The French National Cancer Institute (INCa) accredited the group in 2013, thus acknowledging its academic excellence and operational capability. Since its creation, the group has conducted more than 40 national and international multicenter clinical trials, as well as various translational research projects.


The current research strategy of the French Breast Cancer Intergroup focuses on three major axes:
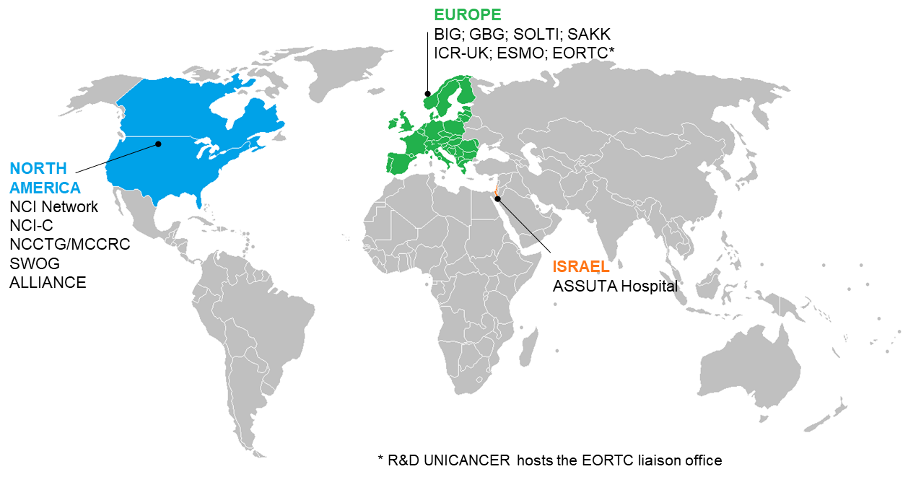
UCBG is increasing its international activities through collaborations with several cooperative groups abroad at the European (BIG, EORTC, UK NCRN, SOLTI, IBCSG, SAKK, GBG) as well as international level (SWOG, ALLIANCE, MCCRC).
UCBG IS BECOMING A KEY INTERNATIONAL PLAYER IN CLINICAL RESEARCH ON BREAST CANCER.
10 studies under recruitment in 2020, 7 of which are international.
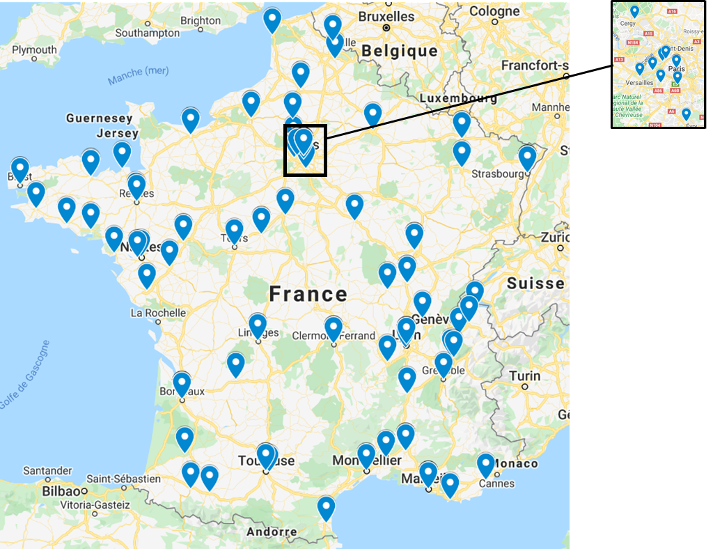
A network of more than 100 investigational centres

More than 2500 patients included in 2020

25 publications or congress presentations in 2020
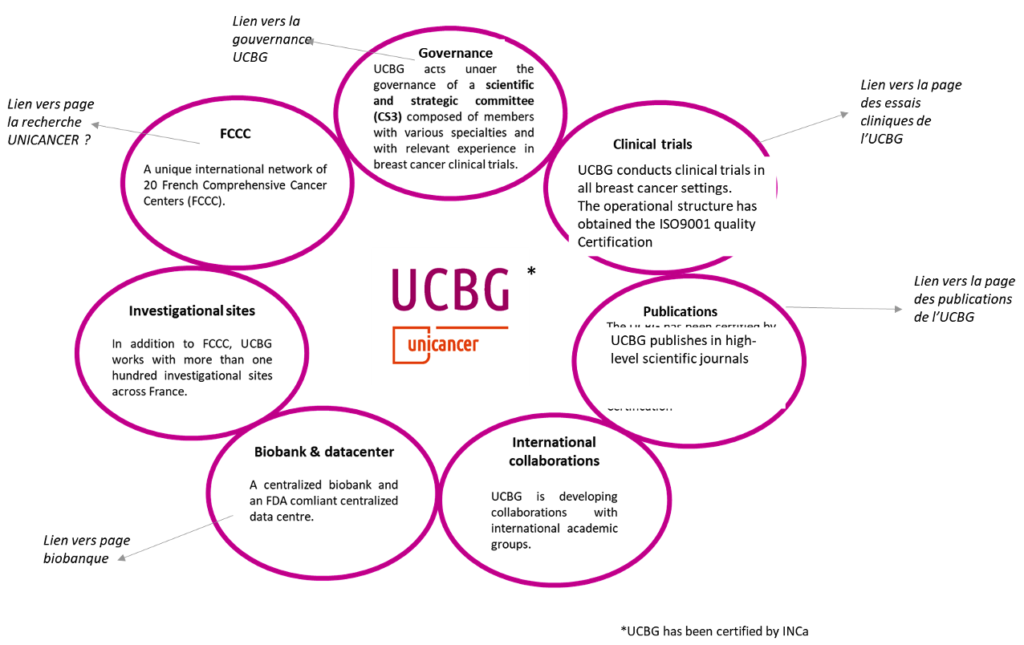
UCBG acts under the governance of a scientific and strategic committee (CS3) composed of members with various specialties and with relevant experience in breast cancer clinical trials. The CS3 includes also two representatives from Patients associations. Members represent the various institutions (FCCC, University Hospital, private centres) participating in clinical trials and in the definition of the Intergroup’s strategy.
The main missions of this scientific committee are to :
The CS3 is composed of a chairperson, a restricted bureau composed of 4 persons (including a vice-chairperson and a secretary), a minimum of 15 members and 2 patients representatives. Approximately 1/3 of the persons belong to an institution outside the FCCC.
Strategic priorities:
Prevention : MyPeBS
MyPeBS (My Personal Breast Screening) is the first European randomised clinical study, conducted on a European scale that aims to evaluate the benefits of a screening programme where the frequency of screening will be adapted to the individual risk of breast cancer for each woman. This study is conducted across multiple centers, in which 85,000 female volunteers between 40 and 70 years old will participate and be randomly placed in one of two groups: a group following standard screening, currently ongoing in the participating countries and a group evaluating a new personalised screening strategy based on the individual risk of breast cancer.
PADA-1
The PADA-1 trial, which has included more than 1,000 patients, is designed to detect the increase of an ESR1 mutation in circulating tumour DNA, collected every two months, in patients receiving first-line treatment with palbociclib and letrozole for metastatic RH+ breast cancer. If the mutation is detected, but there is no clinical sign of progression, patients are randomised to continue on the aromatase inhibitor or to be replaced with fulvestrant while continuing palbociclib.
SAFIR02-Breast
This study aims to demonstrate that the personalised approach, consisting of a treatment adapted to the molecular profile of the tumour, brings a clinical benefit to the patient by delaying the progression of the disease, compared to standard chemotherapy.
Anyone specialised in senology can propose a study. CS3 has all the experts who can help develop your project !
The criteria for acceptance/refusal of a study/trial to be developped within UCBG are transparent and are as follows:
Circuit for the development of new tests/studies
The proposal must be written in the form of a synopsis, by e-mail to the UNICANCER Clinical Programme Lead.
The synopsis should include: title and acronym, objectives, end points, inclusion and exclusion criteria, study design, treatments under study if applicable, scientific background, statistical hypotheses, estimated costs (if available) and type of financial support envisaged.
The synopsis can be written in English (preferably) or French.
Proposals remain confidential within CS3 committee. All CS3 members have signed a confidentiality agreement.
The project leader will be invited to discuss his/her proposal during a CS3 meeting.
After validation by CS3 committee, the trial can be implemented with the help of the UNICANCER R&D project unit.
Personalised medicine
The Unicancer Personalised Medicine Programme develops multidrug predictors to enable the selection of the most effective treatments for individual patients. This multidisciplinary group of experts in biology-driven medicine develops programmes aimed at: proof of concept for personalised treatments, identification of predictors of sensitivity or resistance to therapy, identification of biomarkers of relapse or extreme responses and validation of therapeutic decision algorithms based on biological tests. In 2019, the genomic characterization of metastatic breast cancer has been published in the prestigious journal Nature (Bertucci et al. Nature. 2019; 569 (7757): 560-564)
Survivorship: CANTO
CANTO is a unique long-term follow-up prospective national cohort. It aims to quantify and predict treatment-related chronic toxicities, and to evaluate the psychological, social and economic impact of these toxicities in patients with non-metastatic breast cancer. Over 12,000 women have been included.
TRAK-ER (study not yet started)
International partners:
UCBG is increasing involvement in international trials in collaboration with the main cooperative groups abroad at the European (BIG, EORTC, UK NCRN, SAKK, GBG) as well as international (SWOG, MCCRC) level, UCBG is becoming a key international player in clinical research on breast cancer.
Main French investigators :