- RWD
Unicancer provides support to expert groups responsible for addressing questions on therapeutic strategies and research.

PROMOTING AND DEVELOPING AN INNOVATIVE AND STRATEGIC RESEARCH PROGRAM IN THE FIELD OF RADIATION ONCOLOGY.
The UNITRAD cooperative group was created in 2014. It brings together experts from various disciplines such as radiation oncologists, physicists, dosimetrists, radiobiologists and statisticians. UNITRAD aims to develop strategic guidance in radiation therapy, to set up multidisciplinary collaborative networks in France and abroad, and to stimulate and organize innovative research programs. This group is open to all researchers interested in the development of knowledge in the different fields of radiation oncology.
UNITRAD promotes a research program in oncologic radiation therapy including clinically dedicated radiotherapy, applied radiophysics, brachytherapy, innovative technologies and combinations of ionizing radiation and systemic treatments including targeted therapies and immunotherapy.
UNITRAD as a transverse Group closely interacts with the other Unicancer expert groups, pooling their respective networks as well as their biological, methodological and technical expertise, with the goal to conduct innovative and strategic clinical studies with a strong translational component in a standardized fashion.
Within UNITRAD, five working groups are dedicated to
Several translational projects are under development, in the fields of artificial intelligence, radiobiology and immunology, PROMs/real life data, quality assurance of radiotherapy.

Approximately 100 multidisciplinary experts already joined UNITRAD

7 ongoing clinical trials with over 1500 patients enrolled.
6 communications at international congresses.
UNITRAD is composed of members with various specialties (Radiation Oncologists, physicists, dosimetrists, statisticians, radiobiologists, radiotherapy quality assurance specialists…, ) and with relevant experience in radiation oncology clinical trials. Members represent the various institutions (FCCC, University Hospital, private centres) participating in clinical trials and in the definition of the group strategy.
The main missions of this steering committee are to :
The steering committee is composed of a chairperson, a restricted bureau composed of 4 persons (including a vice-chairperson and a secretary), 5 working groups’ coordinators and a minimum of 15 members.
UNITRAD promotes an active quality approach and collaborates with companies experts in the field of Quality Assurance and artificial intelligence for medical imaging and radiotherapy in order to guarantee a very high level of quality:
HYPOG-01
This a non-inferiority phase III open-label, randomized, controlled multicentric trial comparing hypofractioned versus standard radiotherapy in breast cancer with an indication for regional lymph node irradiation in terms of lymphedema occurrence. From September 2016 to March 2020, 1265 patients have been included in this trial. The first results will be published in 2021.
COLLABORATIVE PROJECT
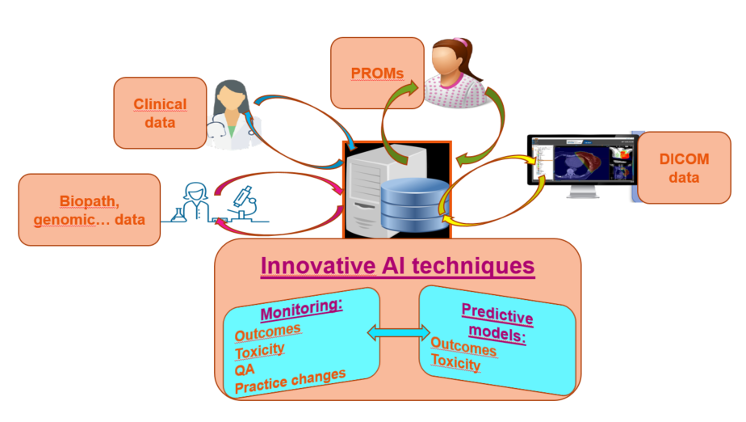
A collaborative project between UNITRAD and Therapanacea, a start-up that spun out of world-renowned centres in radiotherapy and artificial intelligence (AI) research, has been launched in 2019.
The project aims at developing and implementing a new-generation of quality control tool using AI technologies that will enable to automatize, generalize and standardize the verifications of organs-at-risk (OAR) and target volumes delineation for cancer patients treated by radiotherapy in the framework of academic or industrial clinical trials. Currently, verification of delineation is a manual and time-consuming process that can only be performed for a handful of patients cases included in a trial. However, the quality of delineation in those trials is decisive to the following steps of the trials and successful analysis of their results.
This novel tool uses the quality of contouring of Breast anatomy conducted during HYPOG-01 trial. UNITRAD provided data and expertise in order for Therapanacea to develop and validate the QC tool.
The first results of this collaborative project have been presented in international congresses in 2020 (ESTRO/ASTRO).
The successful realization of the project shall lead to a revolution and immediate benefit to all cancer-related clinical trials that include radiotherapy data through massive gain of time, and maximum increase in quality of data injected in the trial, through the ability to apply quality control procedures to all new included patient data without additional effort or cost.
BEPCOME-MB
This is a Phase II, international, randomised, controlled, open-label, multicentric, parallel trial assessing the efficacy and safety of adding upfront stereotactic radiosurgery (SRS) to binimetinib-encorafenib plus pembrolizumab combination therapy in the treatment of BRAFV600 mutation-positive melanoma with brain metastasis. The recruitment is this study is planned in Q4 2021.
The study is supported financially by Pierre Fabre Medicaments.
NIRVANA LUNG
This is an open-labelled, randomized, multicentric phase III study comparing pembrolizumab & chemotherapy with radiotherapy versus pembrolizumab & chemotherapy alone in patients with advanced non-small cell lung cancer. 510 patients are expected to be enrolled. Recruitment in this study is still ongoing.
Microbiota translational research of Nirvana-Lung study is part of Oncobiome project which is supported by the European Union’s Horizon 2020 grant.
Anyone specialised in radiation oncology area can propose a study. UNITRAD steering committee has all the experts who can help develop your project.
The criteria for acceptance/refusal of a study/trial to be developped within UNITRAD are transparent and are as follows:
Circuit for the development of new tests/studies
The proposal must be written in the form of a synopsis, by e-mail to the " target="_blank" rel="noreferrer noopener">U">nicancer Clinical Programme Lead.
The synopsis should include: title and acronym, objectives, end points, inclusion and exclusion criteria, study design, treatments under study if applicable, scientific background, statistical hypotheses, estimated costs (if available) and type of financial support envisaged.
The synopsis can be written in English.
Proposals remain confidential within UNITRAD Steering committee. All members have signed a confidentiality agreement.
The project leader will be invited to discuss his/her proposal during a steering committee meeting or during the annual general assembly.
After validation, the trial can be implemented with the help of the Unicancer project unit.




International partners
UNITRAD is increasing involvement in international trials in collaboration with the main cooperative groups abroad at the European (EORTC, EADO) as well as international (CCTG, ongoing collaboration) level, UNITRAD is becoming a key international player in clinical research on radiation oncology field.