Unicancer provides support to expert groups responsible for addressing questions on therapeutic strategies and research.
Personal data protection at Unicancer
Wishing to advance cancer research, Unicancer has set up numerous initiatives in favour of transparent and secure management of patients’ personal data, whether they are treated within the Unicancer network or included in clinical trials promoted by the Centres or by our R&D department.
Health data protection is essential
The health data of patients carries an important scientific interest for furthering research and developing treatments and the care offer in oncology. Thus, through the development of digital health, these data have become essential to the improvement of the healthcare system and to answering to scientific research challenges.
The increased interest in this type of data exposes them to various risks which need to be managed by the bodies authorised to handle them. By adopting the General Data Protection Regulation (GDPR), the European Union has adopted a regulatory safeguard. The use of health data is strictly regulated within a framework, the non-observance of these provisions exposing organisations to very heavy financial penalties.
A high level of security is a premise to any change in terms of innovation, as is the trust of all stakeholders. Patients in particular must be able to be informed and to keep control over their data at all times.
Unicancer’s commitment
Wishing to further advance cancer research, Unicancer is highly committed to the transparent and secure data management of patients treated within our network or participating in a clinical trial (promoted by Unicancer or by a cancer centre).
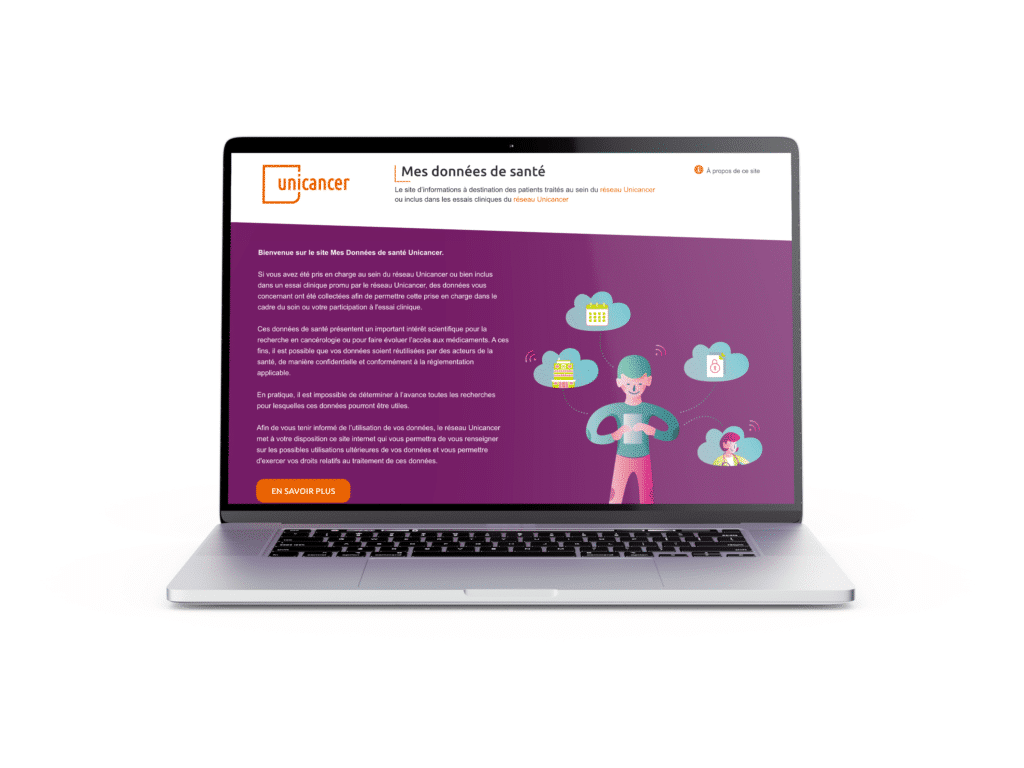
The website “Mes données de santé” (My health data, in French) – used by patients to keep abreast of how their data is used – is the perfect example of the importance we attach to this matter.
This commitment to careful data management and reinforced personal data protection was confirmed in 2018 by the appointment of the first Data Protection Officer (DPO), who was then succeeded by Ms Audrey Acloque, today member of the Unicancer management team.

“Thanks to the website “Mes données de santé” (My health data) we guarantee the compliance of Unicancer’s research with legislation and we can inform patients about the use of their personal data”
Prof. Jean-Yves Blay, Unicancer President
Thanks to the trust from patients, Unicancer today has a large structured database and a collection of biological samples, collected in many indications and pathologies, which is recognised and valued among the scientific community. As it involves sensitive data, Unicancer has set up methodologies to provide a framework for their use and sharing them for the benefit of national and international large scale scientific projects, while ensuring patients’ rights are protected.
Unicancer attaches great importance to the security of the data that patients entrust to the network. To do that, Unicancer ensures its obligations in terms of security are fulfilled, within its teams and in its relationships with its partners. Unicancer has especially undertaken the implementation of a quality management system for the Research and Development Division and the Data and Partnerships Division which were certified compliant with standard ISO 9001for activities relating to the implementation, analysis and use of clinical trials and real-life databases. Unicancer has also launched initiatives related to pharmacovigilance as part of its clinical trials.

The importance of health data within our strategy was also demonstrated by the creation in 2020 of the new Unicancer Health Data and Partnerships Department, by the integration of this subject in Unicancer’s proposals for National health sector reform “Ségur de la santé” and of the French ten-year cancer strategy, and by the implementation of several flagship projects in this area, including the programme ESME (ESME Breast / ESME Ovary / ESME Lung) , the programme Consore, the project OncoDataHub (ODH) and WeShare : to find out more about these initiatives, visit this page.
ESME health data warehouse
Derived from the Epidemiological-Strategic Medico-Economic programme launched in 2014 by Unicancer, the ESME program centralises and structures the real-life data of patients treated in cancer healthcare facilities for breast, ovarian and lung cancer. This warehouse is useful to research teams who have ambitious goals in terms of artificial intelligence applied to oncology.
It was compiled while taking account of the challenges of legislation concerning patient data protection. Compilation of the warehouse and use of its data have been authorised by the French data protection authority (Commission Nationale Informatique et Libertés – Cnil).
Health data research
FAQ
These rights can be exercised by contacting the Unicancer data protection officer:
What is personal data?
Personal data is information used to identify a person directly or indirectly. Identification is possible from a single datum (e.g.: name, DNA, social security number etc.), or from cross-referencing of data (e.g.: an address, age, lifestyle habit etc.).
What is health data?
Health data is personal data known as “sensitive” data. They cover the past, present or future physical and mental states of a person. Through their sensitive nature, their use is prohibited unless expressly provided for by legal provisions (e.g.: scientific research, medical management etc.
What is the GDPR?
Entering into application on 25 May 2018, the acronym GDPR stands for “General Data Protection Regulation”. This European regulation aims to harmonise legislation on data protection over the entire European Union and to reinforce the rights of citizens over their personal data.
What is a health data warehouse?
A data warehouse is a centralised administrative and medical database pooling data from different sources (collected on admissions, consultations, recruitment in a clinical trial and hospitalisation of treated patients) and stored in the same location. This database derives from the need to centralise data for use by the scientific community (research projects, compilation of groups of patients (“cohorts”)
Can people accessing the data warehouse identify patients?
No: Only authorised professionals can access the warehouse as part of research using data and they cannot identify patients. The data are anonymised (de-identified) and secured, which means that information used to identify a patient are deleted or modified so the data cannot be linked to a precise identity. Reuse of the data from the warehouse is subject to a strict regulatory framework.
How are patients informed of how their health data are used?
The health data of patients can be collected and used in several situations:
- When they are treated in a centre in the Unicancer network
The healthcare organisation issues information to the patient on the processing of their data by the nursing team looking after them during the course of treatment for their disease. This information can be disseminated via posters, in the welcome booklet or handed to patients directly.
- On inclusion in a clinical trial sponsored by Unicancer
Unicancer writes an information leaflet and consent form for participation in the study for patients included in the trial it is sponsoring. This information leaflet and consent form also specify the elements related to protection of patient data and rights.
This leaflet is submitted for approval to the ethics committee (Comité de Protection des Personnes - CPP) before it is handed to the patient by the trial investigator.
- On reuse of health data from the Unicancer warehouse or from studies sponsored by Unicancer
Patients can visit the website https://mesdonnees.unicancer.fr/ which lists the scientific projects in which their data were used again. They can also exercise their rights using the form on the website.
How long is the data kept for?
Storage time varies depending on what the data are used for. Storage times are subject to a strict regulatory framework.
Where are the patients’ data stored?
All the data from the Unicancer databases (clinical research, data warehouses) are hosted in France, with a certified health data host.
Are patients’ personal data transferred outside the European Union?
In partnerships the data may be transferred to recipients outside the EU. Unicancer ensures that the recipient has all the necessary guarantees to ensure the protection of patients’ data.
The patients are informed of transfer of their data and can exercise their right to oppose transmission at any time (see Q8).
Data sharing is subject to a contract setting out strict rules of use and security measures to be implemented to be able to access them.
What rights do patients have?
Patients have rights when their data are collected for the first time and when they are reused, and they can exercise those rights at any time, without having to provide reasons
- right to access the patient’s personal data;
- right to correct incorrect or incomplete data;
- right to delete patient data;
- right of limitation.
Exercising any of these rights shall not affect the patient’s care in the healthcare organisation looking after them.
Exercising any of these rights shall not affect the patient’s care in the healthcare organisation looking after them.
These rights can be exercised by contacting the Unicancer Data protection officer :
- by e-mail:dpo@unicancer.fr
- by post: Unicancer, Data protection officer, 101, rue de Tolbiac - 75654 Paris Cedex 13 (France).



